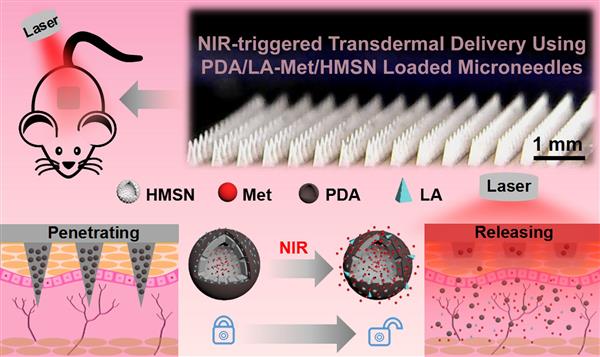
Polymeric microneedles integrated with metformin-loaded and PDA/LA-coated hollow mesoporous SiO2 for NIR-triggered transdermal delivery on diabetic rats
writer:Yang Zhang, Guohua Jiang,* Wenjie Hong, Menyue Gao, Bin Xu, Jiangying Zhu, Gao Song, Tianqi Liu
keywords:microneedles; diabetes; NIR irradiation; transdermal delivery; hypoglycemic
source:期刊
specific source:ACS Applied Bio Materials, DOI: 10.1021/acsabm.8b00470
Issue time:2018年
Herein, a NIR-responsive polymeric microneedles (MNs) system incorporated with metformin-loaded and polydopamine/lauric acid (PDA/LA)-coated hollow mesoporous SiO2 have been developed for transdermal delivery of antidiabetic drug (metformin). Firstly, an anti-diabetic drug was firstly loaded within hollow mesoporous SiO2 nanoparticles (HMSN) by a diffusion method. Then, PDA as photothermal conversion agent and lauric acid (LA) as phase change material (PCM) were coated onto the HMSN to form NIR-responsive drug nanocarriers. Finally, these metformin-Loaded and PDA/LA-coated HMSN were encapsulated into poly(vinylpyrrolidone) (PVP) MNs. After insertion into skin tissue, LA could be melt with the photothermal conversion of PDA under NIR-light, and thus enabling to release encapsulated metformin from MNs. The in vivo release behavior of metformin from MNs into skin was further studied to investigate its hypoglycemic effect on diabetic rats. Compared with the subcutaneous injection of metformin, the bioavailability of MNs-NIR groups was 95.8±2.7%. The antidiabetic drug can be precisely released by adjustment of exposure time and power densities of NIR-light.