[2011][J. Mater. Chem., 2011,21, 7678-7685]A Precursor Strategy for the Synthesis of Low Band–Gap Polymers: An Efficient Route to a Series of Near–Infrared Electrochromic Polymers
writer:Gang Qian, Hana Abu, Zhi Yuan Wang
keywords:Donor-acceptor polymer, Electrochromisim, Near-infrared
source:期刊
specific source:J. Mater. Chem., 2011,21, 7678-7685
Issue time:2011年
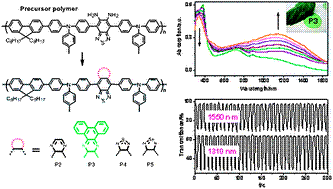
A precursor strategy for the synthesis and screening of a series of conjugated donor–acceptor polymersis demonstrated by successful preparation of low band-gap polymers(P2–P5) containing triphenylamineas anelectron donor and several heterocycles as acceptors, such as[1,2,5]thiadiazolo[3,4-g]quinoxaline,[1,2,5]thiadiazolo[3,4-i]dibenzo[a,c]phenazine, benzo[1,2-c:4,5-c′]bis([1,2,5]thiadiazole), and selenadiazole[3,4-f]benzo[c][1,2,5]thiadiazole, that are transformed from a single reactive polymer(P1). Polymers P2–P5 have the band gap of 1.71–1.29 eV and show the absorption and emission in the near infrared (NIR) spectral region. All the polymers are also NIR electrochromic. In particular, polymer P3 is electrochemically switchable between leaf-like green coloring and near-infrared absorbing states with an efficiency of 479 cm2C?1at 1310 nm or 232 cm2C?1at 1550 nm, making it potentially useful for electrically switchable day-to-night camouflage applications.
全文链接:http://pubs.rsc.org/en/content/articlelanding/2011/jm/c1jm10629e#!divAbstract