The construction of dimensional polymorphic supramolecular polymers driven by adaptive aromatic ring cation-π action is reported in Angew.chem.in.ed.
Nature employs bottom-up self-assembly to create complex biological systems with precise structure and function. Therefore, precise and controllable self-assembly has become a research hotspot in supramolecular chemistry. However, subtle changes in the external environment can seriously affect the final assembly structure, which poses a challenge to predict the molecular self-assembly process to achieve the desired structure in artificial systems. Polymorphism is a typical example, which refers to the ability of molecules to have multiple packing arrangements when they aggregate, and has a profound impact on the structure and physical and chemical properties of the system. It is widely existing and plays an important role in many fields such as chemistry, physics, materials science and biology. Therefore, the preparation of controllable polymorphic supramolecular systems, especially dimensional polymorphisms, is of great significance. However, due to the inherent configurational variability and environmental sensitivity of traditional non-covalent bond interactions, it is still challenging to design specific non-covalent bond modes of interaction to obtain the expected polymorphic supramolecular polymers.
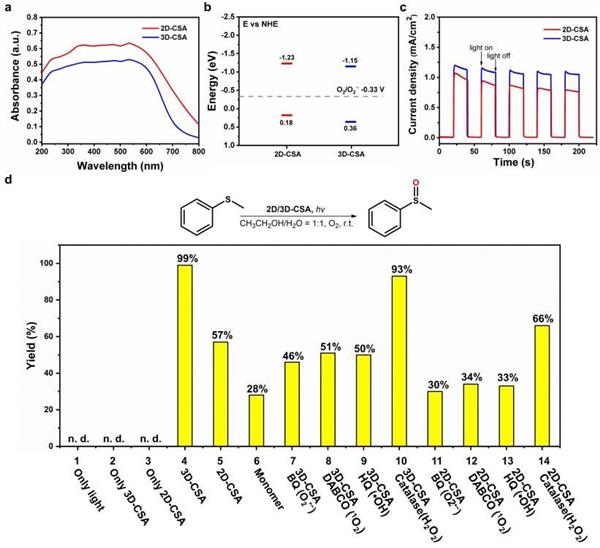
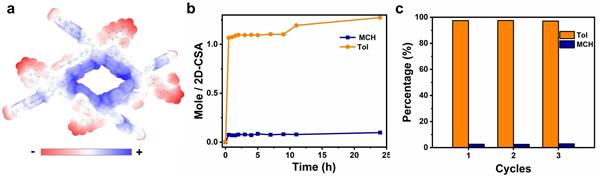
Recently, on the basis of the previous controllable cation-π chemical construction of supramolecular polymers, our research group successfully realized the controllable construction of dimensional polymorphic supramolecular polymers in solid and solution phases by introducing the aromatic ring cation-π interaction with strong adaptive adjustment ability into the monomer structure and using it for guided supramolecular polymerization. In this work, a C2h symmetric monomer (M1) containing N,N '-dimethyl-4,4' -bipyridinium aromatic cyclic cations and carbazolium π units was designed and synthesized. The electron-rich carbazolium units and electron-poor bipyridinium salts promoted the formation of the intermolecular two-site aromatic cyclic cation-π interaction. The two - and three-dimensional supramolecular polymers of M1 were obtained by the guided polymerization of discrete cation-π-cation and long-range cation-π bonding modes in different solvents. At the same time, the formation of secondary assemblies of dimensional polymorphic supramolecular polymers driven by the aromatic ring cation-π interaction was also observed in the solution phase. It is worth noting that the long-range cation-π bonding mode leads to the three-dimensional supramolecular polymer with optimized charge separation and carrier migration, and thus exhibits excellent photocatalytic oxidation activity, with a yield of up to 99% for the conversion of anisole to methylphenylene sulfoxide. Under the same conditions, the yield of 2D supramolecular polymer is only 57%. On the other hand, the two-dimensional supramolecular polymers showed higher toluene/methylcyclohexane separation efficiency (96.9%) because the bipyridinium cation plane in the pores was perpendicular to the two-dimensional plane, which promoted the recognition and adsorption of toluene. The selective adsorption capacity of three-dimensional supramolecular polymer for toluene/methylcyclohexane is relatively low. These results not only provide ideas for the design of new supramolecular systems through "controllable cation-π chemistry", but also open up a new way for the diversified application of supramolecular functional materials.

The relevant research results were published in Angewandte Chemie International Edition (Angew.Chem.Int.Ed. 2024, e202402760).
Doctoral student Zhang Juan and master student Chao Yi are the co-first authors of the paper, and Professor Tian Wei from Northwestern Polytechnical University is the corresponding author of the paper.