The research work on "Design and Construction of Anticancer Hypoxia-Responsive Ruthenium(II) Coordination Supramolecular Metallocomplexes" has been published online in Advanced Functional Materials
The rapid proliferation of tumor tissue, coupled with the abnormal structure of tumor blood vessels, creates a highly hypoxic environment within the tumor. This hypoxic state poses significant challenges for oxygen-dependent therapies, including chemotherapy, photodynamic therapy, and radiation therapy. Currently, a commonly adopted approach to combat tumor hypoxia involves co-delivery of chemotherapeutic drugs alongside O2 catalytic materials. However, this strategy presents limitations such as low loading capacity, reduced reusability, drug leakage during transport, and decreased efficiency in generating in situ O2 within cancer cells. In light of this, our research group proposes a novel strategy that combines organic metal coordination compounds with supramolecular interactions to achieve efficient and stable in situ O2 self-supply within cancer cells, effectively addressing tumor hypoxia.
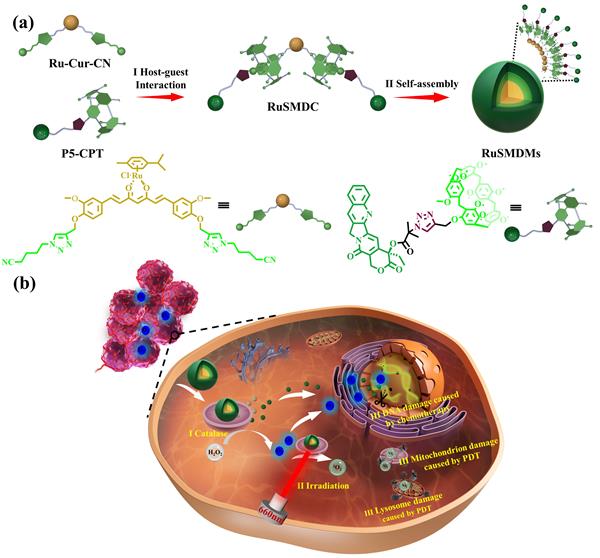
Firstly, we designed and synthesized core molecules (P5-CPT) based on camptothecin drugs, with pillararene moieties at the terminals, and guest molecules (Ru-Cur-CN) based on curcumin drugs coordinated with ruthenium and containing cyanide groups (Figure 1a).The ruthenium in Ru-Cur-CN can catalyze the overexpressed H2O2 in cancer cells, generating a large amount of O2 in the cancer cells. Secondly, the host-guest recognition between pillararene and cyanide groups is utilized to form supramolecular metallocomplexes with ruthenium(II) coordination (Figure 1aⅠ). These complexes can self-assemble into supramolecular spherical micelles containing ruthenium in water (Figure 1aⅡ). Finally, in vivo and in vitro biological experiments have confirmed that these micelles can improve the tumor hypoxic microenvironment, enhance the synergistic anti-tumor effects of chemotherapy and photodynamic therapy (Figure 1bⅢ), and reduce the toxic side effects on normal organs.
Theresearch findings have been published online in "Advanced Functional Materials".
(Paper link: https://onlinelibrary.wiley.com/doi/10.1002/adfm.202105837).
Doctoral student Chengfei Liu from our research group and Muqiong Li from the Air Force Medical University are the co-first authors of the paper, and Associate Professor Li Fan and Professor Wei Tian from the Air Force Medical University are the corresponding authors of the paper.