25.【J. Appl. Polym. Sci.】Aggregation behavior of symmetric poly(n-butyl acrylate)-block-poly(acrylic acid) on subphases of different ionic strengths
writer:Shicheng Yang, Gangyao Wen*, Stergios Pispas, Kun You
keywords:Langmuir-Blodgett film; Block copolymer; Polyelectrolyte; PnBA-b-PAA
source:期刊
specific source:Journal of Applied Polymer Science 2022, 139(29), e52641.
Issue time:2022年
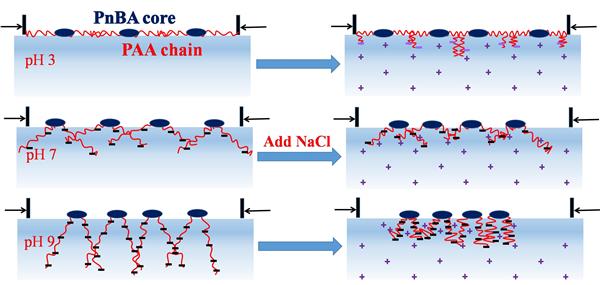
Influences of subphase pH value and ionic strength (NaCl concentration) on the aggregation behavior of a symmetric diblock polyelectrolyte poly(n-butyl acrylate)-block-poly(acrylic acid) (PnBA-b-PAA) at the air/water interface and its Langmuir-Blodgett (LB) films were studied. With the increase of subphase pH value, surface pressure-molecular area isotherms of PnBA-b-PAA monolayers shift toward smaller area due to the gradually enhanced ionization and underwater solubility of hydrophilic PAA chains. All the initial LB films transferred from different subphases with/without NaCl (before monolayer compression) exhibit the densely mixed structures of worm-like aggregates and circular micelles, which indicates that the aggregate formation belongs to the spontaneous mechanism. The micelle core diameters in the LB films increase with the increase of subphase pH value, owing to the enhanced PAA chain solubility and PnBA block aggregation. Under acidic condition, micelle cores are slightly larger upon addition of NaCl due to the enhanced underwater solubility of the uncharged PAA chains triggered by their possible interaction with Cl^_. Under neutral/alkaline conditions, micelle cores are also larger upon addition of NaCl, which is attributed to the electrostatic shielding effect of Na^+. Upon compression, the LB films prepared under neutral/alkaline conditions exhibit the predominantly large circular micelles.
Article address: https://doi.org/10.1002/app.52641.