15.【Polymer】Effects of spreading and subphase conditions on the interfacial behavior of an amphiphilic copolymer poly(n-butylacrylate)-b-poly(acrylic acid)
作者:Shicheng Yang, Gangyao Wen*, Stergios Pispas, Kun You.
关键字:LB film, Block copolymer, Polyelectrolyte, PnBA-b-PAA, Spreading condition
论文来源:期刊
具体来源:Polymer 172 (2019) 66–74
发表时间:2019年
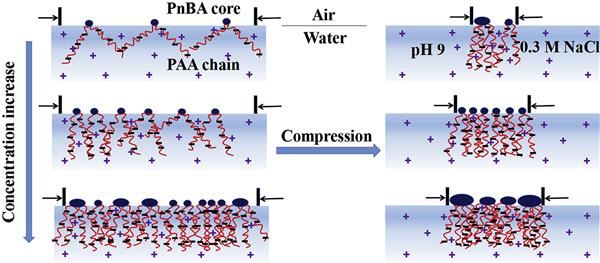
Aggregation behavior of an amphiphilic diblock polyelectrolyte poly(n-butylacrylate)-b-poly(acrylic acid) (PnBA-b-PAA) at the air/water interface and its Langmuir-Blodgett (LB) films were respectively characterized by the Langmuir film balance technique and atomic force microscopy. Effects of spreading solution concentration and volume, and subphase temperature and ionic strength on the interfacial aggregation behavior of PnBA-b-PAA under alkaline condition were systematically studied. With the increase of spreading concentration, surface pressure-molecular area isotherms shift to small molecular areas at the corresponding spreading volumes. With the rise in subphase temperature, the isotherms shift to small molecular areas due to the decreased pKa value and increased solubility of PAA blocks. With the increase of subphase ionic strength, the isotherms shift to large molecular areas because of the predominant salting out effect. The LB films transferred from alkaline subphase with different ionic strengths are more likely to exhibit isolated circular micelles. With the increase of spreading concentration, diameters of micelle cores first decrease and then increase, a behavior which is attributed to the gradually increased chain entanglements and surface polymer concentration, respectively. For small spreading volume, core diameters in the LB films first decrease and then increase with the rise in subphase temperature. For large spreading volume, however, core diameters increase with temperature due to the higher mobility and weaker entanglements of PAA blocks underwater. With the increase of subphase ionic strength, furthermore, core diameters first increase and then decrease, which is attributed to the predominant electrostatic shielding and salting out effects, respectively.
Article address: https://www.sciencedirect.com/science/article/pii/S0032386119302915