Synthesis and versatile postpolymerization modification of couplable A(BC)mD heterografted comblike block quaterpolymers
Xiao Jiang, Wei Shao, Kun Jiang, Meijing Zhang, Huanhuan Liu, Chunnun Ye and Youliang Zhao*
http://pubs.rsc.org/en/content/articlelanding/2013/py/c3py00217a
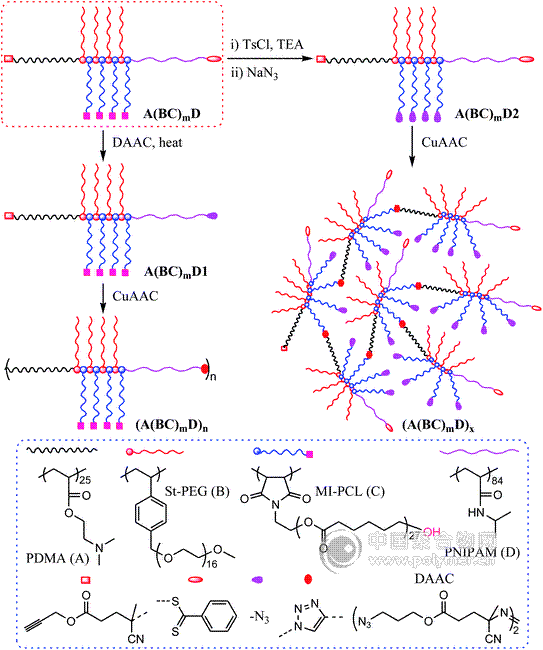
The synthesis and postfunctionalization of A(BC)mD (A = PDMA; B = PEG; C = PCL; D = PNIPAM, PtBA and PMA) comblike block copolymers comprising alkyne and tertiary amino functionalities and alternating PEG and PCL grafts are described. Three-step successive RAFT processes using prop-2-ynyl 4-(benzodithioyl)-4-cyanopentanoate as an original mediator were used to synthesize amphiphilic PDMA(PEG-alt-PCL)mPM (m ≈ 7) heterografted copolymers, in which the PEG and PCL segments were introduced by the alternating copolymerization of styrenic and maleimidic macromonomers. GPC-MALLS and 1H NMR results indicated that the resultant quaterpolymers were of well-controlled molecular weight and relatively low polydispersity (PDI = 1.12–1.25). The combination of end group transformation and the azide–alkyne “click” reaction afforded (A(BC)mD)n (n ≈ 4) comblike-linear multiblock copolymer and (A(BC)mD)x (x ≈ 7) dendritic graft copolymer, and Menschutkin reaction between A(BC)mD copolymer and bromide-functionalized small molecule/macromolecule gave an ion-bearing A′(BC)mD comblike block copolymer and A(BC)mD-graft-E toothbrushlike copolymer, revealing the great potential of postpolymerization modification in novel architecture construction. Preliminary results indicated that the introduction of various functionalities and polymer segments into brushlike copolymers via the quaternization process could affect the chain relaxation and melting behaviors, solubility, and surface wettability of polymer films.