癌症由于其固有的异质性和动态演变,仍然是一种难以治疗的疾病。其治疗迫切需要能够克服肿瘤异质性和动态演变的药物输送系统,以实现药物在肿瘤组织进行动态深层远端递送。

近日,来自法国国家科研中心马赛纳米科学跨学科中心(CINaM,CNRS)的彭玲博士团队在PNAS发表题为Dendrimer nanosystems for adaptive tumor-assisted drug delivery via extracellular vesicle hijacking (PNAS, 2023, Vol. 120, No. 7, e2215308120)的论文,报告了一种基于自组装树形分子纳米载药胶束(ADNMs)通过现场劫持内源性细胞外囊泡(EVs)的方式,克服肿瘤内微环境其异质性和动态演变, 深层递送抗癌药物 (图1), 表现出出色的抗癌活性。
细胞外囊泡是一种内源性的运输系统,是细胞间进行物质与信号交流的重要工具。几乎所有的细胞类型都分泌细胞外囊泡。细胞外囊泡具有纳米和微米尺寸,可以装载各种内源性的蛋白质和核酸,以及外源性材料。重要的是,细胞外囊泡在进化上是保守的,而在肿瘤中是过度产生的。在肿瘤内原位产生的细胞外囊泡不仅可以实现靶向运输,还能够随着肿瘤微环境中的母体细胞一同进化, 是一个理想的药物输送系统,能够适应异质性和进化的肿瘤微环境,同时深入肿瘤进行有效的深层远端递送。
文中报道,自组装树形分子纳米胶束在到达肿瘤后,诱导肿瘤分泌的细胞外囊泡,并将所携带的药物重新包装进入细胞外囊泡。这些原位生成的细胞外囊泡被其他细胞进一步摄取和运输(图2),将药物送入肿瘤组织深处,提高了药物输送效率和癌细胞杀伤力(图3)。在胰腺癌和结直肠癌的二维、三维和异种移植小鼠模型中, 表现了出色的抗癌效力,同时消除了药物的不良影响 (图3)。
该研究成果凸显了自组装树形分子纳米系统作为药物递送载体的巨大潜力,为癌症药物的适应性递送提供了新的思路,即利用肿瘤的固有特征与自组装树形分子超分子化学一起开发智能、高效的药物输送系统,以克服肿瘤内微环境其异质性和动态演变,从而改善癌症治疗,最终提高癌症治疗的效果。

Figure 1: Amphiphilic dendrimer nanomicelles (ADNMs) encapsulate the anticancer drug and induce tumor-assisted drug delivery via extracellular vesicle (EV)-mediated intercellular transport. The amphiphilic dendrimer (AD) encapsulates the anticancer drug and forms nanomicelles (ADNMs) which reach the tumor lesion via the enhanced permeability and retention (EPR) effect. There they induce in situ tumor-assisted drug delivery for deep tumor penetration via EV-mediated intercellular transport. This EV-mediated delivery process involves: (1) internalization of ADNMs inside cells within the tumor tissue; (2) repackaging of ADNM payload into EVs; (3) intercellular transport of the generated EVs; (4) internalization of the generated EVs by the recipient cell.
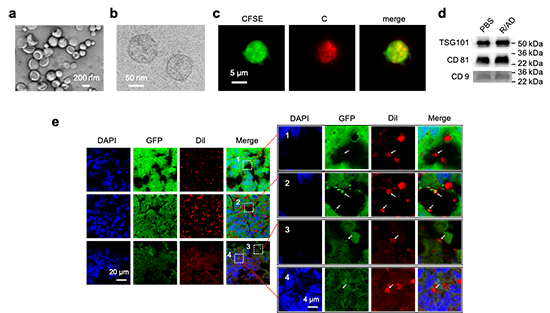
Figure 2. ADNM induced EV payload-packaging and cellular uptake. EVs, generated by cells upon treatment with ADNMs (R/AD, Cy3/R/AD and Dil/R/AD), were characterized using TEM (a), cryogenic electron microscopy (Cryo-EM) (b), fluorescent microscopy (c) and EV using western blotting (d). e, Confocal images of cryo-sectioned tumor tissues from HCT-8GFP xenograft mice treated with ADNM (DiI/R/AD) show the process of EV-mediated delivery in the tumor. The DiI fluorescence appeared in EVs derived from HCT-8GFP tumor (arrows). The hollow-donut shape of red DiI signal with green GFP filling highlights the HCT-8GFP-derived EVs with the DiI-labelled phospholipid bilayer and the HCT-8GFP-derived contents inside. Box 1, a DiI-loaded EV (arrow) located within the intercellular space; Box 2–4, the DiI-loaded EVs mediated intercellular transport within the tumor. Box 2, DiI-loaded EVs adhered onto the cell surface; Box 3, a DiI-loaded EV entering into a cell; Box 4, a DiI-loaded EV inside a cell. The tumor tissues were collected 24 hours after intravenous injection of DiI/R/AD in HCT-8GFP xenografts. AD: amphiphilic dendrimer; C: Cy3; Dil: fluorescent dye; R: rapamycin.
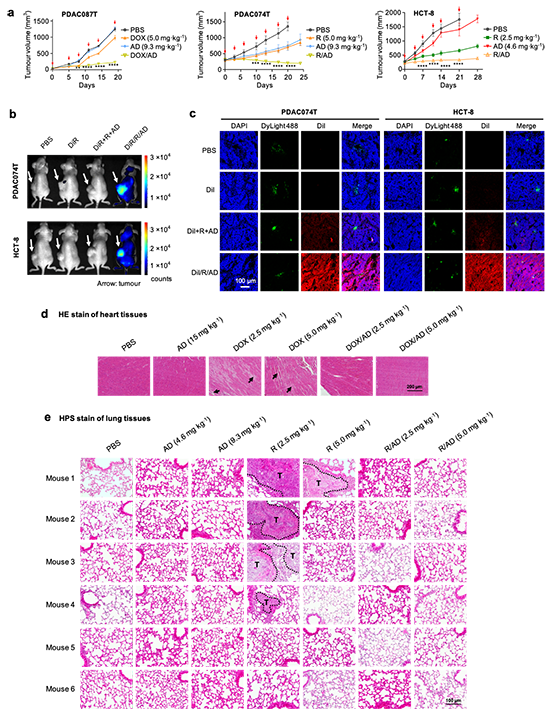
Figure 3. ADNMs were effective for inhibiting tumor growth, reducing drug toxicity and preventing tumor metastasis via specific accumulation and deep penetration through EVs in tumor. a, Tumor growth curves of the patient-derived pancreatic cancer xenografts PDAC087T and PDAC074T, and the colorectal cancer HCT-8 xenografts in mice upon treatment with ADNM carrying either doxorubicin (DOX) or rapamycin R, respectively. Mice treated with PBS buffer, drug alone or dendrimer alone were used as the controls. Data are presented as the mean?±?s.e.m. The statistical significance was calculated by two-way ANOVA with a Tukey''s multiple comparisons test. n?=?6 mice for all groups. *P?<?0.05, **P?<?0.01, ***P?<?0.001, ****P?<?0.0001. b, Accumulation of R/AD in tumors in the PDAC074T and HCT-8 xenograft mice was analyzed using fluorescent imaging with ADNM carrying both rapamycin and the near-infrared fluorescent dye DiR (DiR/R/AD). The in vivo fluorescence images were acquired 48 hours after intravenous administration of DiR/R/AD in PDAC074T (upper panel) and HCT-8 (lower panel) xenografts. Mice treated with PBS, free DiR, and a simple mixture of DiR, rapamycin and AD (DiR+R+AD), were used as controls. Arrows point to the tumor locations. c, Confocal images of tumor tissues show a deep intratumoral penetration of R/AD, using ADNM loaded with both rapamycin and the fluorescent dye DiI (DiI/R/AD). Mice treated with PBS, DiI alone, or a simple mixture of DiI, rapamycin and AD (DiI+R+AD), were used as controls. Tumors were harvested from PDAC074T (left) and HCT-8 (right) xenografts 24 hours post-intravenous administration, then cryosectioned and imaged by tracing DiI fluorescence (red). Blood vessels were labelled with DyLight488-labelled Lycopersicon esculentum lectin (green), and nuclei with DAPI (blue). d, HE stain of heart tissue issued from PDAC087T xenografts treated with PBS, AD, DOX and DOX/AD (n = 6). Treatment with DOX/AD prevented DOX-induced hyperemia and myocardial fiber breakage (arrows) in heart. e, HPS stain of lung tissues issued from HCT-8 xenografts treated with PBS, AD, R and R/AD (n = 6). Treatment with R/AD prevented the rapamycin-induced lung metastasis (T represents the tumor). Lung metastases were observed in mice treated with R, but not in those treated with PBS, dendrimer alone (AD) or the R/AD. Lung tissue was collected from mice at the end of treatment.
这项研究由法国马赛纳米科学跨学科中心(CINaM)彭玲博士主导,合作者包括马赛癌症研究中心(CRCM)Juan Iovanna博士和中国国家纳米科学与技术中心(NCNST)梁兴杰教授团队,本项工作得到了法国国家抗癌联盟, 中法科技合作蔡元培项目和欧盟纳米科技项目的支持。
本文的第一作者是姜一帆博士,主要致力于药物的靶向输送并开发药物传递的新途径,用以生物影像和药物递送等方面的应用。
彭玲博士是自组装超分子树形分子化学的先驱,开创了自组装树形分子在药物递送和生物医学方面的研究,并成功开发了一系列自组装树形分子超分子纳米体系用于递送抗癌药物 (PNAS 2023, 120, e2215308120; Chem. Commun. 2018, 54, 5956-5959; PNAS 2015, 112, 2978–2983), 核酸药物 (PNAS 2023, 120, e2220787120 ; Nat. Protoc. 2021, 16, 327–351; Nano Research, 2021, 14, 2247–2254) 和显像剂 (PNAS 2018, 115, 11454-11459; Chem. Commun. 2020, 56, 301-304 ; Small 2020, 16, 2003290),为生物医用材料领域的发展开辟了新方向 (Acc. Chem. Res. 2020, 53, 2936-2946; Acc. Mater. Res. 2022, 3, 484-497).
原文链接:https://doi.org/10.1073/pnas.2215308120
- 复旦大学占昌友教授团队:羟基PEG可规避人群预存抗PEG抗体 - 助力LNP高效递送 2024-10-30
- 中国海大孔明、烟大陈大全《Int. Biol. Macromol.》:牛膝多糖“引药下行”双重响应纳米药物递送治疗类风湿关节炎 2023-02-25
- 西工大戴亮亮、南洋理工赵彦利团队等 Nano Today综述:用于调控肿瘤微环境正常化的纳米药物递送系统 2023-02-01
- 浙江大学周民教授团队《ACS Nano》:工程化微藻细胞外囊泡通过调控线粒体稳态预防放射性皮炎 2025-07-26
- 中国科大张青川教授、吴尚犬教授团队 Nano Lett.:通过细胞的“自我激励”高效生产功能性sEV 2025-05-08
- 安中医王琪/程志非/桂双英团队 ACS Nano:基于工程化细胞外囊泡的纳米制剂协调神经炎症和免疫稳态以增强帕金森病治疗 2024-08-22
- 西安交大吴道澄团队 ACS Nano:分子堆砌@无限配位聚合物复合纳米粒实现肿瘤连续高强度光热-热动力交替循环治疗和化疗 2025-08-01
